Pharmaceutico-Analytical Study of Bhuvaneshwara Rasa
DOI:
https://doi.org/10.21760/jaims.v6i5.1487Keywords:
Bhuvaneshwara Rasa, Atisara, Standardization, TLC.Abstract
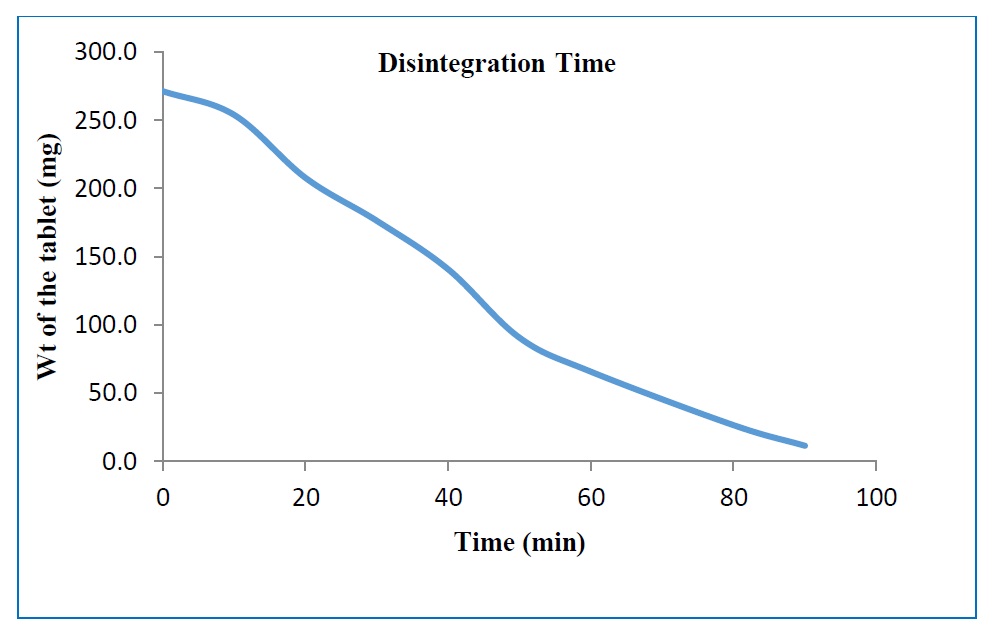
Background: Bhuvaneshwara Rasa is an Ayurvedic preparation mentioned in Bhaishajya Ratnavali used in the treatment of all types of Atisara (diarrhea). There is a lack of data regarding the standardization of pharmaceutical process and analytical profile of Bhuvaneshwara Rasa. Aim: To prepare Bhuvaneshwara Rasa and analyse it using various physicochemical parameters. Materials and methods: Bhuvaneshwara Rasa was prepared as per the guidelines mentioned in Ayurvedic Pharmacopoeia of India. During the pharmaceutical procedure, all the ingredients were mixed thoroughly and triturated with jala. The pharmaceutical and analytical parameters were compiled, and data was recorded. Results: Bhuvaneshwara Rasa after preparation showed increase of 10% yield. The values of physicochemical parameters of Bhuvaneshwara Rasa were as follows: pH 5.69, loss on drying 6.4%, acid insoluble ash 2.5, total ash 8.72%, hardness 3.5 kg, friability 0.1%.TLC band blue at 254nm revealed. Conclusion: Data generated from pharmaceutical, analytical studies and TLC can be used to develop a preliminary standard profile for the formulation Bhuvaneshwara Rasa.
Downloads
References
Shri Das Govind, Bhaishajya Ratnavali, Hindi Translation by Shri Shastri Kaviraja Ambikadatta Ayurveda acharya, Chaukhambha Sanskrit Sansthan, Varanasi; 2005,7th Chapter, Sloka no – 149&150, Pg 234.
Mahendra bhogik. Dhanvantari nighantu. Edited by prof. P.V.Sharma, translated by Dr.Guruprasad Sharma.4th edition. Varanasi: Chaukamba orentelia ; 2004, shatapushpadi varga, verses25, pg.74.
Hegde PL, Harini A. A text book of Dravya guna vijnana. 1st edition. Vol.1.New Delhi: Chaukhambha Publications, 2011: pg.428- 429.
Hegde PL, Harini A. A text book of Dravya guna vijnana. 1st edition. Vol.2.New Delhi: Chaukhambha Publications, 2041: pg.324- 325.
Hegde PL, Harini A. A text book of Dravya guna vijnana. 1st edition. Vol.2.New Delhi: Chaukhambha Publications, 2014: pg.108- 111.
Vaidya VN, Tatiya AU, Elango A, Kukkupuni SK, Vishnuprasad CN. Need for comprehensive standardization strategies for marketed Ayurveda formulations. J Ayurveda Intergr Med.2018:9:312-315.