A controlled clinical trial to evaluate the efficacy of Tilanala Kshara in the management of Vatashteela vis-a-vis Benign Prostatic Hyperplasia
DOI:
https://doi.org/10.21760/jaims.7.10.16Keywords:
Vatashteela, BPH, Prostate, Tilanala Kshara.Abstract
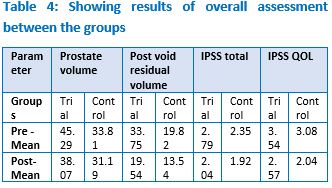
Background: Benign prostatic hyperplasia is the most common urological disease among elderly men, which is characterized by hypertrophic nodular changes of the prostate gland, which is located at the base of the bladder surrounding the proximal urethra. Gland grows at the rate of 2.4cm3/year after the age of 40 owing to the change in hormonal environment. In India, the prevalence rate is around 50% in men above the age of 65 years. The condition can be corelated to Vatashteela in Ayurveda based on the symptomatology. Objective of the study: To evaluate the efficacy of Tila Nala Kshara in the management of Muthraghata vis-a-vis BPH. Materials and Methods: A double arm open labelled clinical study with pre-post-test design was carried out at Government Ayurveda Medical College and Hospital, Mysuru. Data was collected as per the proforma prepared for the purpose of the study. Tila Nala Kshara was administered as intervention in 54 subjects and the control drug was Gokshuradi Guggulu. The data was analysed using SPSS descriptive and inferential statistics. Results: Among the objective parameters, change in prostate volume was significant between the groups with a P value 0.024. But for postvoid residual volume, the difference between the groups was not statistically significant. For subjective parameters which includes IPSS total and Quality of life scale, the difference between the groups was statistically nonsignificant. Conclusion: Tilanala Kshara when used along with Gokshuradi Guggulu was observed to improve general condition of the subjects to greater extent, which clearly implies the added effect of the trial drug.
Downloads
References
Sushruta, Sushrutha Samhitha, with Dalhana’s Nibandha Sangraha commentary, edited by Vaidya Yadavji Trikamji and Narayana Ramacharya, Choukhamba Krishnadas Acadamy Varanasi, 2004. Su. ut -58/27 p789
R.P.Mathur et al, Role of alpha blockers in hypertension with BPH,2014, Journal of The association of Physicians of India, Reapraisal of alpha blockers in hypertension, Vol.62,Page no.40-44
Norman.S.Williams, P.Ronan O.Connell, Andrew.W.Mccaskie , Bailey and love’s, short practice of surgery, 27th Edition 2018, chapmann and Hall, ELBS London, Page no.1458
Acharya JT, editor, 2005, Nibandhasangraha Commentary of Sri Dalhanacharya on Sushrutha Samhita of Sushrutha, Uthara Tandra; Muthraghata Pratishedha: chapter 58, verse 27. Varanasi: Chaukhambha Orientalia Publisher, 2005, p789
Shastri.B.S, editor, 1973, Vidyotini Hindi commentary by Vaidya Lakshmipathi sastri on Yogaratnakara Utharardha, Ashmari chikitsa, Samanya vidhi, Kshara prayoga, verse -1, Varanasi: Chaukhamba Sanskrit Sansthan ,1973,p73
Vidyasagar.P.S, editor, 2013, Adhamalla’s Dipika & Kashirama’s Gudhartha Dipika commentaries on Sharangadhara Samhita of Acharya Sharangadara -Madyamakhanda, chapter 7: Gugguluvidhanam, verse 84-87, Varanasi: Chaukhamba Sanskrit Sansthan, 2013, p.204,205
K.Rajesh, V.Anurag Krishna Tila: A Rasayana Dravya, The pharma innovation Journal
Agnivesha, Charaka Samhita with Chakrapani’s Ayurveda Deepika Commentary, edited by Vaidya Yadavji Trikamji, Chaukhambha Krishnadas Acadamy, Varanasi, 2006 Vi. 8/122p 280