Phytochemical and Antimicrobial Screening of Proprietary Ayurvedic Medicine - N-Dopa Tablet
DOI:
https://doi.org/10.21760/jaims.9.4.7Keywords:
Parkinson's disease, N-Dopa tablet, Quality control, Standardization, Heavy metals, Microbial limit test, Accelerated Stability testingAbstract
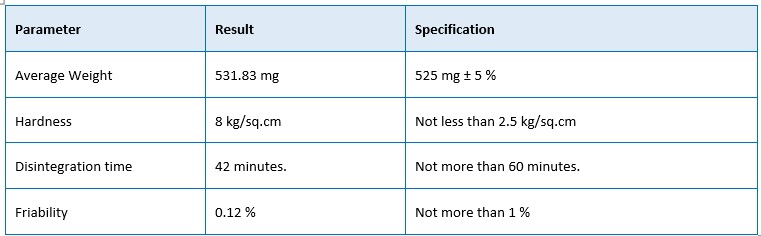
The N-Dopa tablet is a proprietary Ayurvedic poly-herbal formulation widely used in Parkinson's disease, chronic fatigue syndrome, Myalgia and Muscular dystrophy. This study aims to standardize the formulation using advanced analytical techniques and pharmacopoeia standards. The physicochemical testing, heavy metal analysis and microbiological limit test evaluations were carried out in accordance with Ayurvedic pharmacopoeia of India. All the heavy metals were determined to be within the acceptable ranges. The extractive value revealed increases in water soluble extractive, which suggests higher bioavailability in a water medium. In addition, the microbial load was found to be safe for ingestion by humans in terms of microbes. This assessment could aid in determining the drug's authenticity and provide pertinent data for the safer and more effective use of this formulation in therapeutics. These methods will assist drug manufacturers in adhering to regulations and substantiating the stability, safety, and therapeutic efficacy of their goods.
Downloads
References
Zhou ZD, Yi LX, Wang DQ, Lim TM, Tan EK. Role of dopamine in the pathophysiology of Parkinson’s disease. Translational Neurodegeneration. 2023 Sep 18;12(1):44.
Mukherjee PK, Harwansh RK, Bahadur S, Banerjee S, Kar A, Chanda J, Biswas S, Ahmmed SM, Katiyar CK. Development of Ayurveda–tradition to trend. Journal of ethnopharmacology. 2017 Feb 2; 197:10-24.
Suman K, Verma PR, Mazumder R, Monika K, Sheetal C, Deepika T. Standardization an studies on commercial tablets of Valdecoxib.
Chavan S, Tayade S, Gupta V, Deshmukh V, Sardeshmukh S. Pharmaceutical standardization and physicochemical characterization of traditional ayurvedic marine drug: incinerated conch shell (shankha bhasma). Marine drugs. 2018 Nov 15;16(11):450.
Pharmacopoeia I. Government of India, ministry of health and family welfare. Delhi: Controller of Publications. 1996;2(35):448.
Ayurvedic pharmacopoeia of India, 2001, part1, Vol-VI, appendices3.1.3, Pg no.- 291.
Ayurvedic pharmacopoeia of India, 2001, part1, Vol- VI, appendices 2.2.3, Pg no.- 242.
The Ayurvedic pharmacopoeia of India, 2001, part1, Vol- VI, appendix 2.2.4, Pg 243.
Ayurvedic pharmacopoeia of India, 2001, part1, Vol- VI, appendices 2.2.8, Pg no.- 243.
Ayurvedic pharmacopoeia of India, 2001, part1, Vol- VI, appendices 2.2.10, Pg no.- 243.
Garg M, Singh J. Quantitative AAS stimation of heavy metals and trace elements in marketed ayurvedic churna preparations in India. International Journal of Pharmaceutical Sciences and Research. 2012 May 1;3(5):1331.
Ayurvedic pharmacopoeia of India, 2001, part1, Vol- VI, appendices 2.4, Pg no.- 266.
Guideline IH. Stability testing of new drug substances and products. Q1A (R2), current step. 2003 Feb;4(1-24).
Baragi UC, Baragi PC, Vyas MK, Shukla VJ. Standardization and quality control parameters of Dashanga Kwatha Ghana tablet: An Ayurvedic formulation. International journal of Ayurveda research. 2011 Jan;2(1):42.