Assessing the efficacy of Nagarjunabhra Rasa with 21 Bhavna in the management of First-Degree AV Block and Bradycardia: A Comprehensive Case Study
DOI:
https://doi.org/10.21760/jaims.9.10.51Keywords:
Hrid Roga, Nagarjunabhra Rasa, First Degree AV BlockAbstract
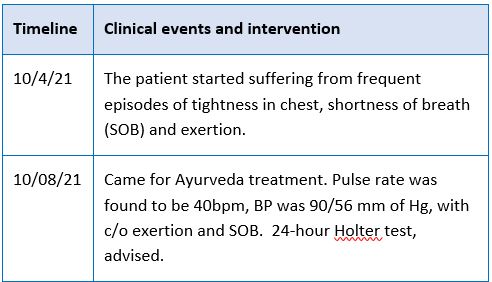
First-degree AV block features a P-R interval over 0.2 seconds, often indicating early AV nodal disease. While it can be benign, especially in individuals with high vagal tone, fibrotic changes are common in older adults. A case study involving a patient with first-degree AV block and a 2.34-second sinus pause treated with Nagarjunabhra Rasa (Arjuna bark’s decoction) showed promising results. Administered as one 125 mg tablet twice daily for three months, the treatment normalized heart rate and eliminated sinus pauses without adverse effects. This suggests the formulation's potential efficacy, warranting further study in larger populations.
Downloads
References
David DS, Gordon FT. The Bradyarrythmias: Disorders of the Atrioventricular Node. In: Jameson JL,Kasper DL,Longo DL, Fauci AS, Hauser SL,Loscalzo J(eds). Text Book of Harrison’s Principles of Internal Medicine, 20th Edition, Vol 2, Mcgraw-Hill Education: New Delhi, 2018, pp1727-1732.
Cheng S, Keyes MJ, Larson MG, McCabe EL, Newton-Cheh C, Levy D et al. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA. 2009 Jun 24;301(24):2571-7. doi: 10.1001/jama.2009.888.
Lewalter T, Pürerfellner H, Ungar A, Rieger G, Mangoni L, Duru F. INSIGHT XT study investigators. "First-degree AV block-a benign entity?" Insertable cardiac monitor in patients with 1st-degree AV block reveals presence or progression to higher grade block or bradycardia requiring pacemaker implant. J Interv Card Electrophysiol. 2018 Aug;52(3):303-306. DOI:10.1007/s10840-018-0439-7.
Agnivesha, Charaka, Dridhabala. Chikitsa Sthana. Chapter 26, Verse: 79-103. In: Shastri KP, Chaturvedi PG (eds). Charaka Samhita of Agnivesha, Reprint edition. Chaukhamba Bharati Academy: Varanasi, 2008, pp 731-736.
Maharshi Susrut. Nidansthana.Chapter1, Verse:17. In: Shastri AD (ed). Susruta Samhita with Hindi translation, Reprint edition. Chaukhambha Sanskrit Sangsthan: Varanasi, 2007, pp 230.
D S Govind. Hridroga Chikitsa Parkaranam; Chapter 33, Verses: 36 –38. In: Ambika Datta Shastri(ed). Bhaishjya Ratnawali: Vidyotini Bhashateeka, Chaukhamba Publication: Varanasi, 2011. P. 692.
Naresh Dalal Et Al: Clinical Efficacy of An Ayurvedic Formulation Nagarjunabhra Rasa In A Post-Mi Patient: Case Study. International Ayurvedic Medical Journal {online} 2017 {cited February, 2017} Available from: http://www.iamj.in/posts/images/upload/393_398.pdf
Maulik SK, Katiyar CK. Terminalia arjuna in cardiovascular diseases: making the transition from traditional to modern medicine in India. Current Pharmaceutical Biotechnology 2010 Dec; 11(8):85560.
Vagbhattachrya. Hridyarog Nidan, Chapter14, Verse: 6-6. In: Tripathi ID (ed). Rasa Ratna Samuchchaya, Rasprabha Hindi Commentary, Reprint edition. Chaukhamba Sanskrit Bhawan: Varanasi, 2003. p. 167.
Rana A. P et all. 2017, The Effective Management of Ventricular Arrythmias with Integrated Ayurvedic Intervention: A Case Study. Int J Recent Sci Res. 8(8), pp. 19035-19037. DOI: http://dx.doi.org/10.24327/ijrsr. 2017.0808.0616
Agnivesha, Dridabala, Charaka, Chakrapani. Viman Sthana, Chapter 1, Verse: 21(2). In: Acharya YT (ed). Ayurved Deepika commentary on Charak Samhita, Chuakambha Surabharati Prakashan: Varanasi, 2011. P. 235.