Transdermal Drug Delivery System: A Review of Current Advances and Challenges
DOI:
https://doi.org/10.21760/jaims.9.11.29Keywords:
Transdermal drug delivery system, physical and chemical method, challengesAbstract
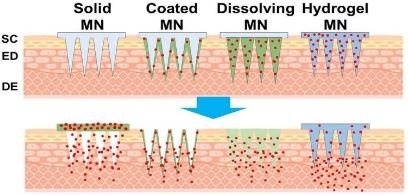
Recently, various non-invasive administrations have emerged as alternatives to traditional needle injections. A Transdermal drug delivery system (TDDS) is the most attractive of these due to its low Rejection rate, excellent ease of administration, and superb convenience and persistence among patients. TDDS Could be applicable not only in pharmaceuticals but also in the skin care industry, which includes cosmetics. Since this This method mainly involves local administration. The skin infusion enhancer technique has been advanced to improve the bioavailability of the drugs. So various Transdermal dosage forms have been prepared like: Transdermal patches, Gel, Cream, Ointments, etc. The Transdermal route is a viable option to enhance the variety of drugs. Transdermal drug delivery has become the primary route of delivery for a variety of medications that would otherwise be difficult to supply. There are some Advantages to Transdermal medicine administration. Mainly to avoid first-pass metabolism and a stomach Environment that would make the drug ineffective in drugs prescribed for skin-related problems and for systemic Effects in curing other organs’ diseases. Hormone replacement therapy, pain relief, smoking withdrawal, Neurological disorders and angina pectoris such as Parkinson’s disease are all under the categories of Transdermal products and Applications. Formulated to release the drug into systemic circulation at the optimal rate, it must be retained in Skin for the required period without inducing sensitization or irritation of the skin. Avoiding first-pass metabolism to achieve Bioavailability with minimal peaks and troughs, Tolerance and dose are being achieved. In the case of Continuous Delivery, maintaining high compliance of the patients is required.
Downloads
References
Akhter N, Singh V, Yusuf M, Khan RA. Non-invasive drug delivery technology: development and current status of transdermal drug delivery devices, techniques, and biomedical applications. Biomed Tech. 2020;65(3):243-72.
Roohnikan M, Laszlo E, Babity S, Brambilla DA. Snapshot of transdermal and topical drug delivery research in Canada. Pharmaceutics. 2019;11(6):256.
Lee H, Song C, Biak S, Kim D, Hyeon T, Kim DH. Device-assisted transdermal drug delivery. Adv Drug Deliv Rev. 2018;127:35-45.
Awuchi CG, Amagwula IO, Priya P, Kumar R, Yezdani U, Khan MG. Aflatoxins in foods and feeds: a review on health implications, detection, and control. Bull Environ Pharmacol Life Sci. 2020;9:149-55.
Kharat RS, Bathe RS. A comprehensive review on: transdermal drug delivery system. Int J Biomed Adv Res. 2016;7:147-59.
Rulea ALM, Perissinato AG, Lino MEDS, Mudrik PS, Pereira GR. Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz J Pharm Sci. 2016;52(3):527-44.
Bathe R, Kapoor R. Transdermal drug delivery system: formulation development and evaluation – an overview. Int J Biomed Adv Res. 2015;6(1):1-10.
Savale SK. A review on transdermal drug delivery system. Asian J Res Biol Pharm Sci. 2015;3(4):150-61.
Prabhakar V, Agarwal S, Sharma S, Sharma S. Transdermal drug delivery system: review. Int Res J Pharm. 2012;3(5):50-53.
Patil PM, Chaudhri PD, Patel JK, Kedar KA, Katolkar PP. Recent trends in challenges and opportunities of transdermal drug delivery system. Int J Drug Dev Res. 2012;4:39-50.
Sloan KB, Wasdo S. Designing for topical delivery: prodrugs can make the difference. Med Care Res Rev. 2003;23:763-93.
Cheong HA, Choi HK. Enhanced percutaneous absorption of piroxicam via salt formation with ethanolamines. Pharm Res. 2002;19:1375-80.
Han T, Das DB. Potential of combined ultrasound and microneedles for enhanced transdermal drug permeation: a review. Eur J Pharm Biopharm. 2015;89:312-28.
Brambilla D, Luciani P, Leroux J. Breakthrough discoveries in drug delivery technologies: the next 30 years. J Control Release. 2014;190:9-14.
Benson HAE. Transdermal drug delivery: penetration enhancement techniques. Curr Drug Deliv. 2005;2(1):23-33.
Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2012;64:128-37.
Cevc G, Blume G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim Biophys Acta. 1992;1104(1):226-32.
Verma P, Pathak K. Therapeutic and cosmeceutical potential of ethosomes: an overview. J Adv Pharm Technol Res. 2010;1(3):274-82.
Kogan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interface Sci. 2006;123-126:369-85.
Banga AK, Chien YW. Iontophoretic delivery of drugs: fundamentals, developments and biomedical applications. J Control Release. 1993;25(1-2):1-25.