Antioxidant and Neuroprotective Potential of Mastakam Yoga via HRMS Analysis: A study on Acorus calamus & Centella asiatica
DOI:
https://doi.org/10.21760/jaims.10.6.15Keywords:
Mastakam Yoga, Neuroprotective, Kynurenic acid, Vanillin, High-Resolution Mass SpectrometryAbstract
Introduction: Globally, the prevalence of neurodegenerative disorders like Dementia, Parkinson's disease, and Alzheimer's disease is increasing, highlighting the need for effective natural neuroprotective and antioxidant agents. In Ayurveda, herbs like Centella asiatica (Mandukparni) and Acorus calamus (Vacha) are well known for their neuroprotective and memory-boosting properties. In this study, a unique herbal formulation called Mastakam Yoga (MSY) was prepared with hydroalcoholic extracts of these two herbs in equal proportion. MSY is suggested to have synergistic antioxidant and neuroprotective benefits. The objective of this study is to discover bioactive components in Mastakam Yoga using High-Resolution Mass Spectrometry and assess their role in neuroprotective and antioxidant activities.
Materials and Methods: Mastakam Yoga (MSY) was formulated by combining an equal amount of hydroalcoholic extracts of Acorus calamus and Centella asiatica. The formulation was analysed using High-Resolution Mass Spectrometry (HRMS) for phytochemical profiling. The compounds were then examined through existing research to check for known antioxidant or neuroprotective qualities. Emphasis was placed on compounds known for antioxidant, anti-inflammatory, and neuroprotective effects.
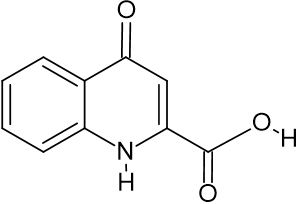
Results: Several bioactive substances, including kynurenic acid, betaine, gabapentin, nootkatone, vanillin, and scopoletin, were found by HRMS analysis of Mastakam Yoga. These chemicals are linked to neuroprotective mechanisms that include oxidative stress inhibition, synaptic plasticity enhancement, and neuroinflammation control.
Discussion: The findings suggest that MSY has strong potential as a natural antioxidant and neuroprotective agent. However, further in vivo and clinical trials are essential to validate these findings and examine the potential therapeutic use of MSY in treating cognitive and neurodegenerative diseases.
Downloads
References
Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem [Internet]. 1992 Nov 1 [cited 2025 May 5];59(5):1609–23. Available from: https://doi.org/10.1111/j.1471-4159.1992.tb10990.x
Kumar GP, Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn Rev [Internet]. 2012 Jul [cited 2025 May 5];6(12):81. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3459459/
Brainina K, Varzakova D, Rudenko YK. Noninvasive electrochemical antioxidant activity estimation: saliva analysis. elibrary.ru [Internet]. 2018 [cited 2025 Apr 27]. Available from: https://elibrary.ru/item.asp?id=35769916
Tramutola A, Lanzillotta C, Perluigi M, Butterfield DA. Oxidative stress, protein modification and Alzheimer disease. Brain Res Bull [Internet]. 2017 Jul 1 [cited 2025 May 5];133:88–96. Available from: https://www.sciencedirect.com/science/article/pii/S0361923016301290
Behl C, Moosmann B. Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach. Free Radic Biol Med [Internet]. 2002 Jul 15 [cited 2025 May 5];33(2):182–91. Available from: https://www.sciencedirect.com/science/article/pii/S0891584902008833
Jiang T, Sun Q, Chen S. Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s and Alzheimer’s disease. Prog Neurobiol [Internet]. 2016 [cited 2025 Apr 27]; Available from: https://www.sciencedirect.com/science/article/pii/S0301008215300071
Teleanu RI, Chircov C, Grumezescu AM, Volceanov A, Teleanu DM. Antioxidant therapies for neuroprotection—A review. J Clin Med [Internet]. 2019 Oct 11 [cited 2025 May 5];8(10):1659. Available from: https://www.mdpi.com/2077-0383/8/10/1659
Horton W, Török M. Natural and nature-inspired synthetic small molecule antioxidants in the context of green chemistry. In: Green Chemistry: An Inclusive Approach [Internet]. 2018 Jan 1 [cited 2025 May 5]. p. 963–79. Available from: https://www.sciencedirect.com/science/article/abs/pii/B9780128092705000327
Perchyonok T, Reher V, Grobler S, Oliver A, Zhang S. Bioactive-functionalized interpenetrating network hydrogel (BIOF-IPN): a novel biomaterial transforming bio-repair, bio-adhesion and therapeutic capability—An in vitro study. J Integr Med Dent Sci [Internet]. 2015 [cited 2025 May 5]. Available from: http://dx.doi.org/10.4172/jimds.1000166
Bhat S, Kamal M, Yarla N, Ashraf G. Synopsis on management strategies for neurodegenerative disorders: challenges from bench to bedside. Curr Top Med Chem [Internet]. 2017 Apr 3 [cited 2025 May 5];17(12):1371–8. Available from: https://www.benthamdirect.com/content/journals/ctmc/10.2174/1568026616666161222121229
Hickenbottom SL, Grotta J. Neuroprotective therapy. Semin Neurol [Internet]. 1998 [cited 2025 May 5];18(4):485–92. Available from: http://www.thieme-connect.com/products/ejournals/html/10.1055/s-2008-1040901
Singh R, Malviya R. Pharmacological properties and Ayurvedic value of Indian Buch plant (Acorus calamus): a short review. J Biol Res [Internet]. 2011 [cited 2025 May 5]. Available from: https://www.researchgate.net/publication/235989568
Balakumbahan R, Rajamani K, Kumanan K. Acorus calamus: an overview. J Med Plants Res [Internet]. 2010 [cited 2025 May 5];4(25):2740–5. Available from: http://www.academicjournals.org/JMPR
Kirtikar KR. Indian Medicinal Plants. Allahabad: Lalit Mohan Basu; 1918.
Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acorus calamus: scientific validation of Ayurvedic tradition from natural resources. Pharm Biol [Internet]. 2007 Oct [cited 2025 May 5];45(8):651–66. Available from: https://www.tandfonline.com/doi/pdf/10.1080/13880200701538724
Singh S, Gautam A, Sharma A, Batra A. Centella asiatica (L.): a plant with immense medicinal potential but threatened. Int J Pharm Sci Rev Res. 2010;(2).
Sharma BL, Kala A. Biodiversity of medicinal plants of Triyugi Narain (Garhwal Himalaya) and their conservation. In: Sharma BL, editor. National Conference on Recent Trends in Spices & Medicinal Plant Research; 1998 Apr 2–4; Calcutta, WB, India. p. A-78.
Gohil KJ, Patel JA, Gajjar AK. Pharmacological review on Centella asiatica: a potential herbal cure-all. Indian J Pharm Sci [Internet]. 2010 Sep [cited 2025 May 5];72(5):546. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3116297/
Abdallah MS, Mustafa M, Nallappan MA, Choi S, Paik JH, Rusea G. Determination of phenolics and flavonoids of some useful medicinal plants and bioassay-guided fractionation of Sclerocarya birrea stem bark extract. Front Chem [Internet]. 2021 Jul 27 [cited 2025 May 5];9:670530. Available from: https://ui.adsabs.harvard.edu/abs/2021FrCh....9..541A/abstract
Martos D, Tuka B, Tanaka M, Vécsei L, Telegdy G. Memory enhancement with kynurenic acid and its mechanisms in neurotransmission. Biomedicines [Internet]. 2022 Apr 5 [cited 2025 May 5];10(4):849. Available from: https://www.mdpi.com/2227-9059/10/4/849/htm
Lugo-Huitrón R, Blanco-Ayala T, Ugalde-Muñiz P, Carrillo-Mora P, Pedraza-Chaverrí J, Silva-Adaya D, et al. On the antioxidant properties of kynurenic acid: free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol Teratol [Internet]. 2011 Sep 1 [cited 2025 May 5];33(5):538–47. Available from: https://www.sciencedirect.com/science/article/pii/S0892036211001498
López-Sánchez C, Martín-Romero FJ, Sun F, Luis L, Samhan-Arias AK, García-Martínez V, et al. Blood micromolar concentrations of kaempferol afford protection against ischemia/reperfusion-induced damage in rat brain. Brain Res [Internet]. 2007 Nov 28 [cited 2025 May 5];1182(1):123–37. Available from: https://www.sciencedirect.com/science/article/pii/S0006899307021336
Li WH, Cheng X, Yang YL, Liu M, Zhang SS, Wang YH, et al. Kaempferol attenuates neuroinflammation and blood–brain barrier dysfunction to improve neurological deficits in cerebral ischemia/reperfusion rats. Brain Res [Internet]. 2019 Nov 1 [cited 2025 May 5];1722:146361. Available from: https://www.sciencedirect.com/science/article/pii/S0006899319304159
Zhang Y, Jia J. Betaine mitigates amyloid-β-associated neuroinflammation by suppressing the NLRP3 and NF-κB signaling pathways in microglial cells. J Alzheimers Dis [Internet]. 2023 [cited 2025 Apr 28]. Available from: https://content.iospress.com/articles/journal-of-alzheimers-disease/jad230064
Ibi D, Tsuchihashi A, Nomura T, Hiramatsu M. Involvement of GAT2/BGT-1 in the preventive effects of betaine on cognitive impairment and brain oxidative stress in amyloid β peptide-injected mice. Eur J Pharmacol [Internet]. 2019 Jan 5 [cited 2025 May 5];842:57–63. Available from: https://www.sciencedirect.com/science/article/pii/S0014299918306228
Qi Y, Cheng X, Gong G, Yan T, Du Y, Wu B, et al. Synergistic neuroprotective effect of schisandrin and nootkatone on regulating inflammation, apoptosis and autophagy via the PI3K/AKT pathway. Food Funct [Internet]. 2020 Mar 26 [cited 2025 May 5];11(3):2427–38. Available from: https://pubs.rsc.org/en/content/articlehtml/2020/fo/c9fo02927c
He B, Xu F, Xiao F, Yan T, Wu B, Bi K, et al. Neuroprotective effects of nootkatone from Alpinia oxyphylla fructus against amyloid-β-induced cognitive impairment. Metab Brain Dis [Internet]. 2018 Feb 1 [cited 2025 May 5];33(1):251–9. Available from: https://link.springer.com/article/10.1007/s11011-017-0154-6
Emmez H, Börcek AÖ, Kaymaz M, Kaymaz F, Durda E, Ivi S, et al. Neuroprotective effects of gabapentin in experimental spinal cord injury. World Neurosurg [Internet]. 2010 Jun 1 [cited 2025 May 5];73(6):729–34. Available from: https://www.sciencedirect.com/science/article/pii/S1878875010001804
Yan BC, Wang J, Rui Y, Cao J, Xu P, Jiang D, et al. Neuroprotective effects of gabapentin against cerebral ischemia reperfusion-induced neuronal autophagic injury via regulation of the PI3K/Akt/mTOR signaling pathways. J Neuropathol Exp Neurol [Internet]. 2019 Feb 1 [cited 2025 May 5];78(2):157–71. Available from: https://dx.doi.org/10.1093/jnen/nly119
Dhanalakshmi C, Manivasagam T, Nataraj J, Thenmozhi AJ, Essa MM. Neurosupportive role of vanillin, a natural phenolic compound, on rotenone-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Evid Based Complement Alternat Med [Internet]. 2015 Jan 1 [cited 2025 May 5];2015:626028. Available from: https://doi.org/10.1155/2015/626028
Malik A, Kushnoor A, Saini V, Singhal S, Kumar S, Yadav YC. In vitro antioxidant properties of scopoletin. J Chem Pharm Res. 2011;3(3):659–65.
Kulkarni R, Girish KJ, Kumar A. Nootropic herbs (Medhya Rasayana) in Ayurveda: an update. Pharmacogn Rev [Internet]. 2012 Jul [cited 2025 May 5];6(12):147. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3459457/