A prospective, multi center, single blind, randomized controlled study evaluating “AyurCoro3” as an adjuvant in the treatment of mild to moderate COVID-19 patients
DOI:
https://doi.org/10.21760/jaims.v6i4.1386Keywords:
Coronavirus infections, COVID-19, Ayurveda, Complementary and Alternative MedicineAbstract
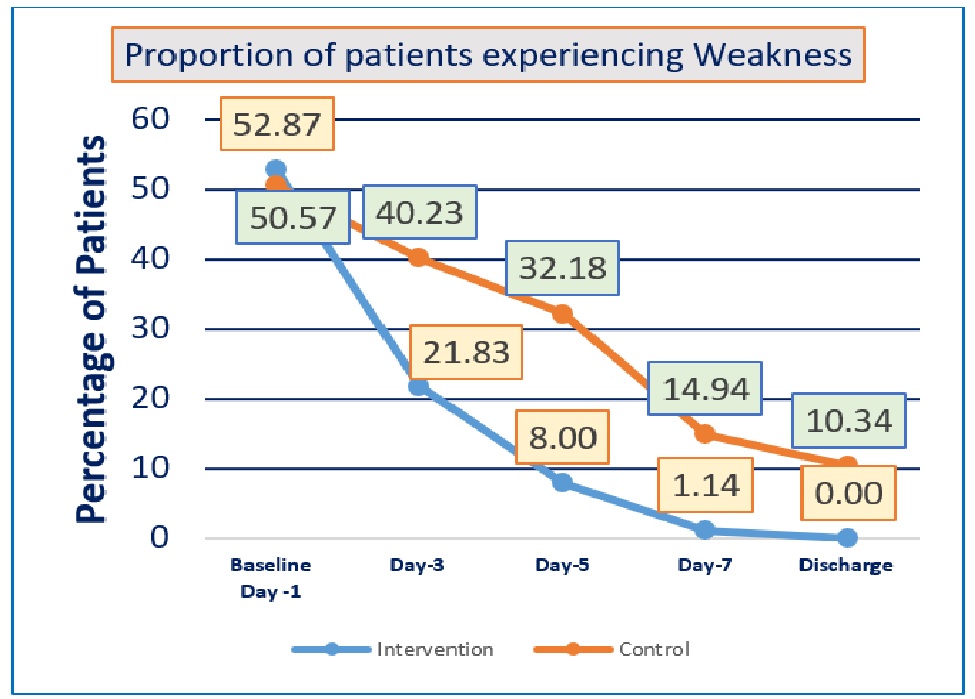
Background: There is so far no proven treatment for the unprecedented COVID-19 infections. Ayurveda holds promise in the treatment of this viral infection. We carried out a randomized controlled trial of ‘AyurCoro-3’, a combination of Gomutra (Bos indicus urine), hot water, turmeric, Turati Churna (potassium Alum), candy sugar (Khadisakhar), Bos indicus milk with two teaspoons of Go Ghrut (Ghee) as an adjuvant to standard care, in comparison to standard care alone in patients with mild-to-moderate COVID-19 infections. Methods: A randomized, blinded, controlled trial was carried out in adult patients diagnosed with mild-to-moderate COVID-19 infections confirmed by reverse transcriptase polymerase chain reaction (RT-PCR) test. Interventional group was administered single dose of ‘AyurCoro-3’ as an adjuvant with standard care, and the control group received only standard of care. Validated clinical improvement scale was used for evaluating the clinical improvement, time of resolution of presenting symptoms, duration of hospitalization, proportion of patients requiring mechanical ventilation, and functional status scale were the key outcomes. Results: One-hundred and seventy-four patients were recruited. Significantly more proportions of patients had resolution of all symptoms (cough, fever, breathlessness, weakness, and tastelessness) in the interventional group compared to control. Similarly, the interventional group also had shorter time for clinical improvement as well as shorter time of resolution for cough, breathlessness, and weakness. No significant differences were observed in the duration of hospitalization, proportion of patients requiring mechanical ventilation, functional status scale, and adverse events between the groups. Conclusion: The Ayurvedic medicine ‘AyurCoro-3’ was observed to significantly shorten the duration of COVID-19 infections and was well tolerated.
Downloads
References
FDA approves first treatment for COVID-19. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (Accessed on 10 May 2021).
Rotti H, Raval R, Anchan S, Bellampalli R, Bhale S, Bharadwaj R, et al. Determinants of Prakriti, the human constitution types of Indian traditional medicine and its correlation with contemporary science. J Ayurveda Integr Med. 2014; 5: 167-75.
Semwal DK, Mishra SP, Chauhan A, Semwal RB. Adverse health effects of tobacco and role of Ayurveda in their reduction. J Med Sci. 2015; 15: 139–46.
Bombardieri D, Easthope G. Convergence between orthodox and alternative medicine: A theoretical elaboration and empirical test. Health. 2000; 4: 479–94.
Chauhan A, Semwal DK, Mishra SP, Semwal RB. Ayurvedic research and methodology: Present status and future strategies. Ayu. 2015; 36(4): 364-369.
Goyal M. Threats and challenges of emerging viral diseases and scope of Ayurveda in its prevention. AYU 2019; 40: 67-8.
Randhawa GK, Sharma R. Chemotherapeutic potential of cow urine: A review. J Intercult Ethnopharmacol. 2015; 4(2): 180-186.
Tijare P, Ambatkar N, Tiwari V. Clinical Improvement In COVID19 Patients With Timely Intervention Of Panchagavya Medicine: A Preliminary Finding. Int J of Pharmc Res [Internet]. 2020; 10(6): e5449.
Jennings MR, Parks RJ. Curcumin as an Antiviral Agent. Viruses. 2020; 12(11): 1242.
Kim KH, Lee YT, Hwang HS, Kwon YM, Jung YJ, Lee Y, Lee JS, Lee YN, Park S, Kang SM. Alum Adjuvant Enhances Protection against Respiratory Syncytial Virus but Exacerbates Pulmonary Inflammation by Modulating Multiple Innate and Adaptive Immune Cells. PLoS One. 2015; 10(10): e0139916.
Clinical management protocol: COVID-19. Government of India, Ministry of Health and Family Welfare, Directorate General of Health Services. Available at: https://www.mohfw.gov.in/pdf/UpdatedClinicalManagementProtocolforCOVID19dated03072020.pdf (Accessed on 3rd July 2020).
CONSORT. Transparent reporting of trials. Available at: http://www.consort-statement.org/ (Accessed on 4th May 2021).
Payyappallimana U, Patwardhan K, Mangalath P, Kessler CS, Jayasundar R, Kizhakkeveettil A, Morandi A, Puthiyedath R. The COVID-19 Pandemic and the Relevance of Ayurveda's Whole Systems Approach to Health and Disease Management. J Altern Complement Med. 2020; 26(12): 1089-1092.
Chauhan RS. Immunomodulatory properties of indigenous cow urine. MOJ Immunol. 2018; 6(5): 302-303.
Thimmulappa RK, Mudnakudu-Nagaraju KK, Shivamallu C, Subramaniam KJT, Radhakrishnan A, Bhojraj S, Kuppusamy G. Antiviral and immunomodulatory activity of curcumin: A case for prophylactic therapy for COVID-19. Heliyon. 2021; 7(2): e06350.