A Prospective, Open-label, Non-randomised Clinical Trial to Evaluate the Safety and Efficacy of Cholecurb in the Treatment of Dyslipidemia
DOI:
https://doi.org/10.21760/jaims.9.7.2Keywords:
Cholecurb Tablet, Dyslipidemia, cardiovascular diseases, metabolic disordersAbstract
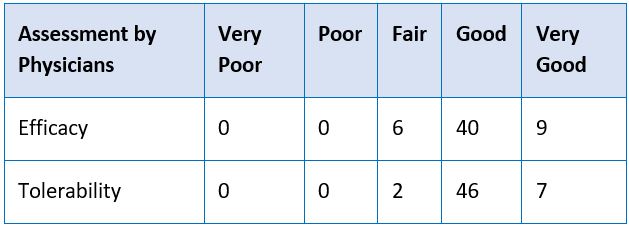
Objectives: To evaluate the clinical efficacy and safety of Cholecurb Tablets in Dyslipidemia. Materials and Methods: A prospective, interventional clinical study was conducted on 55 patients, aged between 18-55 years, confirmed with dyslipidemia and who were willing to give informed consent. All patients received Cholecurb Tablets at a dose of two tablets four times in a day for four weeks. All patients were evaluated at baseline, 45 and 120 days for parameters of fasting plasma lipid profile. The Physician’s global assessment and Patient’s global assessment (at end of the study) on efficacy and tolerability were made on a scale of 1- 5, namely, Very Good = 5, Good = 4, Fair = 3, Poor = 2 and Very Poor = 1. Observation and Results: Cholecurb Tablets significantly improved all lipid outcomes at both 45 days and 120 days after onset of treatment. There were no significant differences in the laboratory findings at baseline, 45 days and 120 days. The global assessment of response by physicians showed that 72% of patients showed a good improvement while another 16% showed very good improvement in their condition by the end of 120 days of treatment. Similarly, 58% and 31% of the patients’ global assessment indicated good and very good response at the end of treatment, respectively. These findings confirm the efficacy of Cholecurb Tablets in the study population during the study period. Conclusion: Cholecurb Tablets typically lowered LDL, TG, TC and elevated HDL, assessed at baseline, 45 days and 120 days. There were no clinically significant adverse events either reported or observed during the entire study period.
Downloads
References
Liu T, Zhao D, Qi Y. Global Trends in the Epidemiology and Management of Dyslipidemia. Journal of Clinical Medicine. 2022; 11(21):6377. https://doi.org/10.3390/ jcm11216377
Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidaemias. Nat Rev Cardiol. 2021 Oct;18(10):689-700.
Mohamed-Yassin MS, Baharudin N, Abdul-Razak S, Ramli AS, Lai NM. Global prevalence of dyslipidaemia in adult populations: a systematic review protocol. BMJ Open. 2021 Dec 03;11(12):e049662.
Madssen, Erik; Laugsand, Lars Erik; Wiseth, Rune; Mørkedal, Bjørn; Platou, Carl; Vatten, Lars; Janszky, Imrec. Risk of Acute Myocardial Infarction: Dyslipidemia More Detrimental for Men than Women. Epidemiology 24(5):p 637-642, September 2013. | DOI: 10.1097/EDE.0b013e31829d2632
Obita George, Alkhatib Ahmad, Disparities in the Prevalence of Childhood Obesity - Related Comorbidities: A Systematic Review, Frontiers in Public Health, 10/2022, DOI.10.3389/fpubh.2022.923744.
Massimiliano Ruscica, Nicola Ferri, Maciej Banach, Cesare R Sirtori, Alberto Corsini, Side effects of statins: from pathophysiology and epidemiology to diagnostic and therapeutic implications, Cardiovascular Research, Volume 118, Issue 17, December 2022, Pages 3288–3304, https://doi.org/10.1093/cvr/cvac020
Patel KK, Sehgal VS, Kashfi K. Molecular targets of statins and their potential side effects: Not all the glitter is gold. Eur J Pharmacol. 2022 May 5;922:174906. doi: 10.1016/j.ejphar.2022.174906. Epub 2022 Mar 20. PMID: 35321818; PMCID: PMC9007885.
Attardo S, Musumeci O, Velardo D, Toscano A. Statins Neuromuscular Adverse Effects. International Journal of Molecular Sciences. 2022; 23(15):8364. https://doi.org/ 10.3390/ijms23158364
Berberich AJ, Hegele RA. A Modern Approach to Dyslipidemia. Endocr Rev. 2022 Jul 13;43(4):611-653. doi: 10.1210/endrev/bnab037. PMID: 34676866; PMCID: PMC9277652.
Rauf, A., Akram, M., Anwar, H. et al. Therapeutic potential of herbal medicine for the management of hyperlipidemia: latest updates. Environ Sci Pollut Res 29, 40281–40301 (2022). https://doi.org/10.1007/ s11356-022-19733-7
Kang YM, Kim YJ, Kim K. Significance of traditional herbal medicine for dyslipidemia. Am J Transl Res. 2023 Aug 15;15(8):5373-5388. PMID: 37692941; PMCID: PMC10492084.
Adel Mehraban MS, Tabatabaei-Malazy O, Rahimi R, Daniali M, Khashayar P, Larijani B. Targeting dyslipidemia by herbal medicines: A systematic review of meta-analyses. J Ethnopharmacol. 2021 Nov 15;280:114407. doi: 10.1016/j.jep.2021.114407. Epub 2021 Jul 9. PMID: 34252530.
Qadeer F, Abidi A, Fatima F, Rizvi DA. Effect of Pterocarpus marsupium in animal model of high carbohydrate diet-induced metabolic syndrome. Natl J Physiol Pharm Pharmacol 2018;8(11):1509-1514.
Pendurkar, S. R., & Mengi, S. A. (2009). Antihyperlipidemic effect of aqueous extract of Plumbago zeylanica roots in diet-induced hyperlipidemic rat. Pharmaceutical Biology, 47(10), 1004–1010. https://doi.org/10.1080/13880200902973779
Aljobouri, Abduljassim & Rashid, Khaleel & Alasadi, Sura & Zyer, Abdulamers & Abbas, Zahraa. (2015). Original Research Article Study the effect of Bauhinia variegate Linn. ethanolic extract on reducing glucose and lipid levels of white albino mice.
Upadya, H., Prabhu, S., Prasad, A. et al. A randomized, double blind, placebo controlled, multicenter clinical trial to assess the efficacy and safety of Emblica officinalis extract in patients with dyslipidemia. BMC Complement Altern Med 19, 27 (2019). https://doi.org/10.1186/s12906-019-2430-y
Mohammadi, A., Sahebkar, A., Iranshahi, M., Amini, M., Khojasteh, R., Ghayour-Mobarhan, M. and Ferns, G.A. (2013), Effects of Supplementation with Curcuminoids on Dyslipidemia in Obese Patients: A Randomized Crossover Trial. Phytother. Res., 27: 374-379. https://doi.org/10.1002/ptr.4715
Zeinab Vafaeipour, Bibi Marjan Razavi, Hossein Hosseinzadeh, Effects of turmeric (Curcuma longa) and its constituent (curcumin) on the metabolic syndrome: An updated review, Journal of Integrative Medicine, Volume 20, Issue 3, 2022, Pages 193-203, ISSN 2095-4964, https://doi.org/10.1016/j.joim.2022.02.008.
Salih, A. K., Alwan, A. H., Khadim, M., Al-qaim, Z. H., Mardanov, B., El-Sehrwy, A. A., Ahmed, Y. I., & Amerizadeh, A. (2023). Effect of ginger (Zingiber officinale) intake on human serum lipid profile: Systematic review and meta-analysis. Phytotherapy Research, 37(6), 24722483. https://doi.org/10.1002/ptr.7769