A prospective, open-label, non-randomised clinical trial to evaluate the safety and efficacy of Rymanyl Tablets in the treatment of Osteoarthritis
DOI:
https://doi.org/10.21760/jaims.9.7.4Keywords:
Rymanyl Tablet, Osteoarthritis, Joint pain, AyurvedaAbstract
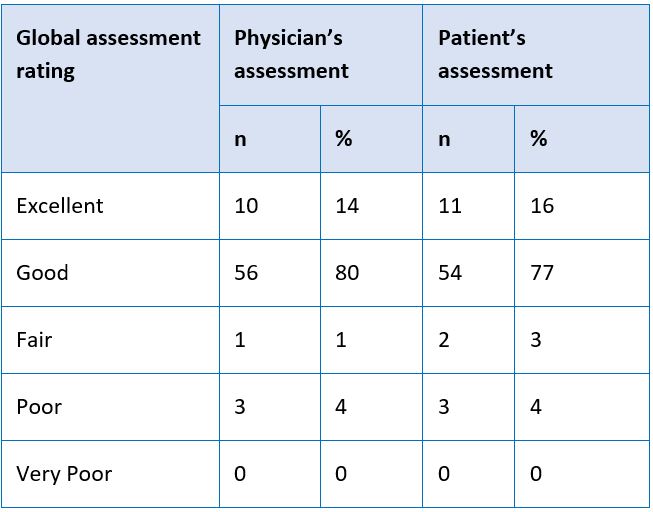
Objectives: To evaluate the clinical efficacy and safety of Rymanyl Tablets in patients with knee osteoarthritis. Material and Methods: A prospective, interventional clinical study was conducted on 70 patients, aged between 18-60 years, confirmed with knee osteoarthritis and who were willing to give informed consent. All patients received Rymanyl Tablets at a dose of two tablets twice in a day for 90 days. All patients were evaluated at baseline and 90 days for improvement in knee flexion, quadriceps and hamstring muscle strength, 6-minute walk test score, European Q5D quality of life; reduction in knee swelling, pain intensity, WOMAC-pain, stiffness and physical function score and VAS pain scale. The Physician global assessment and Patient’s global assessment (at end of the study) on efficacy and tolerability were made on a scale of 1- 5, namely, Excellent = 5, Good = 4, Fair = 3, Poor = 2 and Very Poor = 1. Results: Rymanyl Tablets could significantly improve all outcomes including improvement in knee flexion, quadriceps and hamstring muscle strength, 6-minute walk test score and European Q5D quality of life; reduction in knee swelling, pain intensity, WOMAC-pain, stiffness and physical function score and VAS pain scale. Conclusion: Rymanyl Tablets typically improve knee flexion, quadriceps and hamstring muscle strength, walking capacity, overall physical function and quality of life as well as reduce knee swelling, intensity of pain and stiffness, assessed at baseline and 90 days. There were no clinically significant adverse events either reported or observed during the entire study period.
Downloads
References
Scheuing WJ, Reginato AM, Deeb M, Acer Kasman S. The burden of osteoarthritis: Is it a rising problem? Best Pract Res Clin Rheumatol. 2023 Jun;37(2):101836. doi: 10.1016/j.berh.2023.101836. Epub 2023 Aug 24. PMID: 37633827.
GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023 Aug 21;5(9):e508-e522. doi: 10.1016/S2665-9913(23)00163-7. PMID: 37675071; PMCID: PMC10477960.
https://www.who.int/news-room/fact-sheets/detail/ osteoarthritis
Richard MJ, Driban JB, McAlindon TE. Pharmaceutical treatment of osteoarthritis. Osteoarthritis Cartilage. 2023 Apr;31(4):458-466. doi: 10.1016/j.joca.2022. 11.005. Epub 2022 Nov 19. PMID: 36414224.
Pountos I, Panteli M, Walters G, Giannoudis PV. NSAIDs inhibit bone healing through the downregulation of TGF-β3 expression during endochondral ossification. Injury. 2021 Jun;52(6):1294-1299. doi: 10.1016/j.injury.2021.01.007. Epub 2021 Jan 10. PMID: 33472741.
Latourte, A., Kloppenburg, M. & Richette, P. Emerging pharmaceutical therapies for osteoarthritis. Nat Rev Rheumatol 16, 673–688 (2020). https://doi.org/10.1038/s41584-020-00518-6
Richard MJ, Driban JB, McAlindon TE. Pharmaceutical treatment of osteoarthritis. Osteoarthritis Cartilage. 2023 Apr;31(4):458-466. doi: 10.1016/j.joca.2022. 11.005. Epub 2022 Nov 19. PMID: 36414224.
Santana, É & Lima, V & Souza, J & Quintans, Siqueira & Coutinho, Henrique & Lucetti, E & Tahim, C & Da, W & Júnior, Silva & Quintans-Júnior, Lucindo. (2024). Management of Pain and Inflammation Through Natural Products in Individuals With Knee Osteoarthritis: A Systematic Review. Journal of Herbal Medicine. 45. 100851. 10.1016/j.hermed.2024.100851.
Kalpana Patel, SVVS Ravi Mangu, Shinde Vijay Sukhdeo, Kunal Sharan, Ethanolic extract from the root and leaf of Sida cordifolia promotes osteoblast activity and prevents ovariectomy-induced bone loss in mice, Phytomedicine, Volume 99, 2022, 154024, ISSN 0944-7113, https://doi.org/10.1016/j.phymed.2022.154024.
Pallavi S. Nirmal, Suresh D. Jagtap, Prasad P. Devarshi, Aarti N. Narkhede, Soumya J. Koppikar, Dhanashri R. Ingale, Abhay M. Harsulkar; Cartilage protective effect of Sida cordifolia L. and Piper longum L. is through modulation of MMPs and TIMP; International Journal of Advanced Research (2015), Volume 3, Issue 11, 480 – 488
Nirmal, P.S., Jagtap, S.D., Narkhede, A.N. et al. New herbal composition (OA-F2) protects cartilage degeneration in a rat model of collagenase induced osteoarthritis. BMC Complement Altern Med 17, 6 (2017). https://doi.org/10.1186/s12906-016-1535-9
Ban, Y., Wang, Y., Qiao, L., Zhang, C., Wang, H., He, X., Jia, D., & Zheng, C. (2023). Total lignans from Vitex negundo seeds attenuate osteoarthritis and their main component vitedoin A alleviates osteoclast differentiation by suppressing ERK/NFATc1 signaling. Phytotherapy Research, 37(4), 1422–1434. https://doi.org/10.1002/ptr.7750
Zhang, Li, Li, Xiaochen, Zhang, Haosheng, Huang, Zhengquan, Zhang, Nongshan, Zhang, Li, Xing, Runlin, Wang, Peimin, Agnuside Alleviates Synovitis and Fibrosis in Knee Osteoarthritis through the Inhibition of HIF-1α and NLRP3 Inflammasome, Mediators of Inflammation, 2021, 5534614, 11 pages, 2021. https://doi.org/10.1155/2021/5534614
Gupta, R., Chaudhary, A.K. & Sharma, R. Analgesic and Anti-inflammatory Potential of Ricinus communis Linn.: Evidence from Pharmacology to Clinical Studies. Curr. Pharmacol. Rep. 10, 27–67 (2024). https://doi.org/10.1007/s40495-023-00347-7
G.S.H. Ramakanth, C. Uday Kumar, P.V. Kishan, P. Usharani; A randomized, double blind placebo controlled study of efficacy and tolerability of Withaina somnifera extracts in knee joint pain; Journal of Ayurveda and Integrative Medicine; Volume 7, Issue 3, 2016, Pages 151-157; ISSN 0975-9476; https://doi.org/10.1016/j.jaim.2016.05.003.