Standardization and Quality Assurance of Dadrughni Vati (Lepa) and Dadrughna Malahara: A Critical Quality Control Evaluation
DOI:
https://doi.org/10.21760/jaims.10.2.11Keywords:
Physicochemical analysis, Ayurvedic formulations, Skin care, Dadrughni Vati, Dadrughna MalaharaAbstract
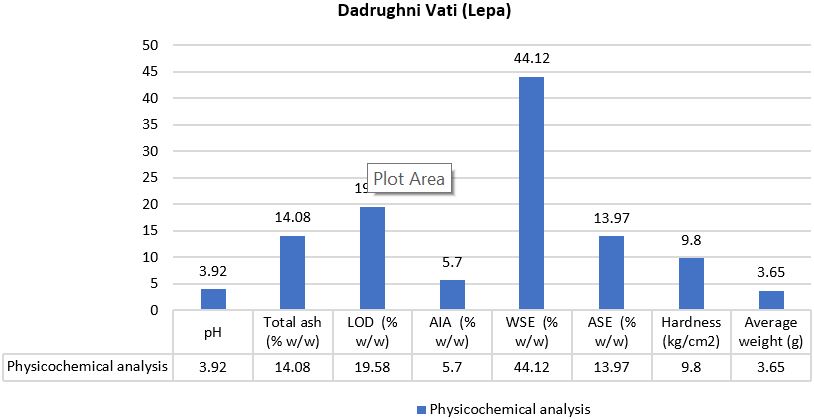
Introduction: Ayurvedic formulations, such as Dadrughni Vati (Lepa) (DL) and Dadrughna Malahara (DM), are used for skin care and treatment. Understanding the physicochemical properties of these formulations is essential to assess their quality, efficacy, and stability. This study aims to evaluate and compare the key physicochemical parameters of DL and DM, focusing on pH, loss on drying (LOD), ash values, extractive properties, and other physical attributes. Methods: The physicochemical analysis of DL and DM was performed using standard analytical procedures. pH was measured using a pH meter; LOD was determined by heating the samples; ash values, including acid-insoluble ash, were quantified through combustion; and water-soluble and alcohol-soluble extractive values were assessed using solvent extraction techniques. Additional physical tests included measuring the specific gravity, acid value, saponification value, iodine value, viscosity, and spreadability. Results: DL exhibited a pH of 3.92, an LOD of 14.08%, and an ash value of 19.58%. Its water-soluble extractive value was 44.12%, and alcohol-soluble extractives were 13.97%. The average hardness was 9.8, and the weight was 3651 mg. DM showed a specific gravity of 0.930, an acid value of 2.22, a saponification value of 118.57, and an iodine value of 77.11. The viscosity and spreadability of DM were 15,64,333 cp and 657.95 g, respectively. Discussion: The physicochemical properties of DL and DM indicate that both formulations are stable, genuine, and suitable for skin application. DL’s pH and extractive values suggest it is moisturizing and mild, making it beneficial for conditions like Dadru, while DM’s specific gravity and emulsifying properties confirm its potential for use as an effective Malahara (skin ointment). The consistency of all parameters across batches further supports the formulation’s reproducibility and quality. These results underscore the therapeutic potential of DL and DM in Ayurvedic dermatological care.
Downloads
References
Study.com. What is Analytical Chemistry? Definition & Impact [Internet]. [cited date]. Available from: https://study.com/academy/lesson/what-is-analytical-chemistry-definition-impact.html.
Gujarat Rajya Bheshaj Samiti. Bheshaj Samhita. Swasthya Mantralaya Gujarat Ahmedabad. Chapter No.13. 1966 ed. p. 745.
Anonymous. The Ayurvedic Pharmacopoeia of India. Part-1, Volume-1, Appendix 2. 1st ed. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of AYUSH; 2009. p. 213.
Anonymous. Laboratory guide for the analysis of Ayurveda and Siddha formulation. 1st ed. New Delhi: GOI, Central Council for Research in Ayurvedic Sciences, Ministry of AYUSH; 2010. p. 27.
Anonymous. The Ayurvedic Pharmacopoeia of India. Part-1, Volume-1, Appendix 2. 1st ed. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of AYUSH; 2009. p. 160.
Anonymous. The Ayurvedic Pharmacopoeia of India. Part-1, Volume-1, Appendix 2. 1st ed. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of AYUSH; 2009. p. 160.
Anonymous. Laboratory guide for the analysis of Ayurveda and Siddha formulation. 1st ed. New Delhi: GOI, Central Council for Research in Ayurvedic Sciences, Ministry of AYUSH; 2010. p. 28.
Anonymous. Laboratory guide for the analysis of Ayurveda and Siddha formulation. 1st ed. New Delhi: GOI, Central Council for Research in Ayurvedic Sciences, Ministry of AYUSH; 2010. p. 28.
Anonymous. The Ayurvedic Pharmacopoeia of India. Part-1, Volume-1, Appendix 2. 1st ed. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of AYUSH; 2009. p. 297.
Anonymous. The Ayurvedic Pharmacopoeia of India. Part-1, Volume-1, Appendix 2. 1st ed. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of AYUSH; 2009. p. 212.
Anonymous. The Ayurvedic Pharmacopoeia of India. Part-1, Volume-1, Appendix 2. 1st ed. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of AYUSH; 2009. p. 223.
Anonymous. The Ayurvedic Pharmacopoeia of India. Part-1, Volume-1, Appendix 2. 1st ed. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of AYUSH; 2009. p. 221.
Anonymous. The Ayurvedic Pharmacopoeia of India. Part-1, Volume-1, Appendix 2. 1st ed. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of AYUSH; 2009. p. 212.
Anonymous. The Ayurvedic Pharmacopoeia of India. Part-1, Volume-1, Appendix 2. 1st ed. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of AYUSH; 2009. p. 222.
The Ayurvedic Pharmacopoeia of India. Government of India, Ministry of Health and Family Welfare, Department of AYUSH. Published by The Controller of Publications, Civil Lines, Delhi; 2008. Appendix 3. p. 221.
Laboratory Guide for the Analysis of Ayurveda and Siddha Formulations. New Delhi: Central Council for Research in Ayurveda and Siddha; 2010. p. 63.
Laboratory Guide for the Analysis of Ayurveda and Siddha Formulations. New Delhi: Central Council for Research in Ayurveda and Siddha; 2010. p. 66.
Kumar PK, Sudhakara M. Formulation and Evaluation of Diclofenac Transdermal Gel. Malla Reddy College of Pharmacy, Andhra Pradesh. 2013 Jul-Sep; p. 250.
Farnsworth NR, et al. Herbal Medicine: From the Laboratory to the Clinic. J Ethnopharmacol. 1996;51(1):1–10.
McCarty et al. Borates: Applications and Analysis. J Chem Educ. 2012;89(6):813.
Madusanka et al. The role of aluminum in water treatment. J Environ Sci Health. 2016.
Krushnakumar et al. Modified methods for Gandhaka Shodhana: A pilot study. J Ayurveda Integr Med. 2016 Mar;2(2):587–593.
Suhasini RD, et al. Pharmaceutico-analytical study of Gandhaka Kalpa. Int Ayurvedic Med J. 2024 Jan;125–130.
Vyas SP. Pharmaceutical development of Jivantyadi Yamaka into Malahara and their comparative clinical efficacy in Ekakustha (Psoriasis) [MD dissertation]. Vadodara: Department of Rasashastra and Bhaishajya Kalpana, Government Ayurvedic College; 2022.
Vadi DA. Pharmaco-therapeutic study to assess comparative efficacy of Chakramarda and Gaumutra Bhavit Chakramarda on Vicharchika [MD dissertation]. Jamnagar: Department of Dravyaguna, IPGT & RA, GAU; 2007.
Houghton PJ, et al. The Role of Moisture Content in Herbal Powder Quality. J Herb Pharmacother. 2002;2(3):27–38.
Baker et al. Thermal analysis of sodium tetraborate decahydrate. Thermochim Acta. 1997;298(1):87–98.
Pavlović V, et al. Characterization of Potash Alum and Its Thermal Behavior. Chem Ind Chem Eng Q. 2016;22(4):515–520.
Suhasini RD, et al. Pharmaceutico-analytical study of Gandhaka Kalpa. Int Ayurvedic Med J. 2024 Jan;125–130.
Vyas SP. Pharmaceutical development of Jivantyadi Yamaka into Malahara and their comparative clinical efficacy in Ekakustha (Psoriasis) [MD dissertation]. Vadodara: Department of Rasashastra and Bhaishajya Kalpana, Government Ayurvedic College; 2022.
Vadi DA. Pharmaco-therapeutic study to assess comparative efficacy of Chakramarda and Gaumutra Bhavit Chakramarda on Vicharchika [MD dissertation]. Jamnagar: Department of Dravyaguna, IPGT & RA, GAU; 2007.
LabMonk. Determination of ash value of given sample [Internet]. [cited date]. Available from: https://labmonk.com/determination-of-ash-value-of-given-sample.
Almeida JFDC, et al. Thermal Behavior of Borax Decahydrate. J Therm Anal Calorim. 2015 Jan;16:67–72.
Dos Santos STMSO, et al. Thermal Decomposition of Potassium Aluminum Sulfate Dodecahydrate. 2008;95(6):617–618.
Kamani V. A Comparative Pharmaceutico-Analytical Study of Rajavarta Bhasma Prepared from Two Different Varieties of Rajavarta [MD dissertation]. Vadodara: Department of Rasashastra and Bhaishajya Kalpana, Government Ayurvedic College; 2023.
LabMonk. Determination of ash value of given sample [Internet]. [cited date]. Available from: https://labmonk.com/determination-of-ash-value-of-given-sample.
Kamani V. A Comparative Pharmaceutico-Analytical Study of Rajavarta Bhasma Prepared from Two Different Varieties of Rajavarta [MD dissertation]. Vadodara: Department of Rasashastra and Bhaishajya Kalpana, Government Ayurvedic College; 2023.
World Health Organization. Quality Control Methods for Medicinal Plant Materials. Geneva: World Health Organization (WHO).
Bhowmik et al. Physicochemical properties of potash alum. Asian J Chem. 2012;24(1):207–212.
Raja M, et al. Thermodynamic Study of Borax: Solubility and Stability in Aqueous Solutions. Int J Res Chem Environ. 2016;6(4):15–20.
Silpa M, et al. A phytochemical study on Eupatorium glandulosum. Asian J Pharm Clin Res. 2020 Jan;13(1):77–80.
Khan MI, et al. Physicochemical properties and extractive values of some medicinal plants. J Med Plants Res. 2013;7(26):1804–1809.
Raja M, Waghmare. Thermodynamic Study of Borax: Solubility and Stability in Aqueous Solutions. Int J Res Chem Environ. 2016;6(4):15–20.
California Skin Institute. What to know about your skin's pH [Internet]. [cited date]. Available from: https://www.californiaskininstitute.com/what-to-know-about-your-skins-ph/#:~:text=Standard%20pH%20Levels.
Yuan Y, et al. Impact of excipients on the bioavailability of topical formulations. Int J Pharm. 2018 Jan;16:67–72.
Liu Y, et al. Influence of formulation components on skin absorption and bioavailability of topical products. Int J Pharm. 2019.
Kumar P, Singh S. Influence of formulation properties on the skin permeation of topical dosage forms. AAPS PharmSciTech. 2020.
Higgins C, et al. The role of fatty acids in skin health and disease. J Clin Dermatol. 2013.
Kumar P, Singh S. Formulation strategies for topical ointments: Importance of saponification value. Asian J Pharm Sci. 2020.
Tiwari G, et al. A review on the stability of emulsions: Factors affecting stability and strategies to improve it. Int J Pharm Sci Res. 2013.