Comprehensive Evaluation of the Pharmacognostic Study and Analytical Parameter of Shigru Kanda Twaka (Stem Bark of Moringa Oleifera Lam.)
DOI:
https://doi.org/10.21760/jaims.10.9.6Keywords:
Shigru, Moringa oleifera Lam.,, Stem bark, Pharmacognosy, Physicochemical, Phytochemical, HPTLCAbstract
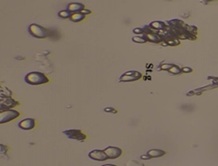
Shigru (Moringa oleifera Lam..) is one of the most important plants having nutritional as well as medicinal value in Ayurveda. This present study has been carried out to establish the stem bark of plant for its morphological, microscopical and physicochemical characters with different phytochemical qualitative tests as per API and Sophisticated analysis such as High-Performance Thin Layer Chromatography (HPTLC). Microscopy of stem bark showed Phloem, Rosette crystal, Resin duct, Stone cell, Medullary rays, Gum secretion, Crystal, Stratified cork. Powder microscopy of Shigru (Moringa oleifera Lam.) stem bark shows starch grain, Crystal fibres, Prismatic crystal, reddish brown tears of gum mass, Rosette crystal. Physicochemical parameters showed pH (6.28), loss on drying (6.28 %w/w), ash value (7.12 %w/w), Acid insoluble ash (1.05 %w/w), water soluble extractive (37.28 %w/v) and alcohol soluble extractive (19.10 %w/w). Preliminary phytochemical analysis for the presence of various functional groups such as Alkaloids, Flavonoids, Tannin, Saponin, Steroids were also studied. These observations can be helpful for identification and standardization of Shigru (Moringa oleifera Lam.).
Downloads
References
Government of India, Ministry of Health and Family Welfare, Department of AYUSH. The Ayurvedic Pharmacopoeia of India. Part 1, Vol. 4. New Delhi: Controller of Publications; p. 114.
Sharifudin SA, Fakurazi S, Hidayat MT, Hairuszah I, Moklas MA, Arulselvan P. Therapeutic potential of Moringa oleifera extracts against acetaminophen-induced hepatotoxicity in rats. Pharm Biol. 2013;51(3):279–88.
Hegde PL. A Textbook of Dravyaguna Vijnan. Vol. 2. New Delhi: Chaukhamba Publications; 2022. p. 646.
Government of India, Ministry of Health and Family Welfare, Department of AYUSH. The Ayurvedic Pharmacopoeia of India. Part 1, Vol. 4. New Delhi: Controller of Publications; p. 114.
Government of India, Ministry of Health and Family Welfare, Department of AYUSH. The Ayurvedic Pharmacopoeia of India. Part 1, Vol. 4. New Delhi: Controller of Publications; p. 114.
Government of India, Ministry of Health and Family Welfare, Department of AYUSH. The Ayurvedic Pharmacopoeia of India. Part 1, Vol. 4. New Delhi: Controller of Publications; p. 114.
Government of India, Ministry of Health and Family Welfare, Department of AYUSH. The Ayurvedic Pharmacopoeia of India. Part 1, Vol. 4. New Delhi: Controller of Publications; p. 114.
Government of India, Ministry of Health and Family Welfare, Department of AYUSH. The Ayurvedic Pharmacopoeia of India. Part 1, Vol. 4. New Delhi: Controller of Publications; p. 114.
Government of India, Ministry of Health and Family Welfare, Department of AYUSH. The Ayurvedic Pharmacopoeia of India. Part 1, Vol. 4. New Delhi: Controller of Publications; p. 114.
Government of India, Ministry of Health and Family Welfare, Department of AYUSH. The Ayurvedic Pharmacopoeia of India. Part 1, Vol. 4. New Delhi: Controller of Publications; p. 114.
Government of India, Ministry of Health and Family Welfare, Department of AYUSH. The Ayurvedic Pharmacopoeia of India. Part 1, Vol. 4. New Delhi: Controller of Publications; p. 114.
Government of India, Ministry of Health and Family Welfare, Department of AYUSH. The Ayurvedic Pharmacopoeia of India. Part 1, Vol. 4. New Delhi: Controller of Publications; p. 114.
Government of India, Ministry of Health and Family Welfare, Department of AYUSH. The Ayurvedic Pharmacopoeia of India. Part 1, Vol. 4. New Delhi: Controller of Publications; p. 114.
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Abhishek Gabani, Suman Singh, Dharmendra Jani

This work is licensed under a Creative Commons Attribution 4.0 International License.














