A Prospective, Open-label Non-Randomized Clinical Trial to evaluate the Safety and Efficacy of M2-Tone Tablet in Treatment of Dysmenorrhea
DOI:
https://doi.org/10.21760/jaims.10.6.2Keywords:
Dysmenorrhea, M2-Tone Tablet, Herbal medicine, Menstrual cramps, Polyherbal formulation, Clinical trial, Pain management, Menstrual healthAbstract
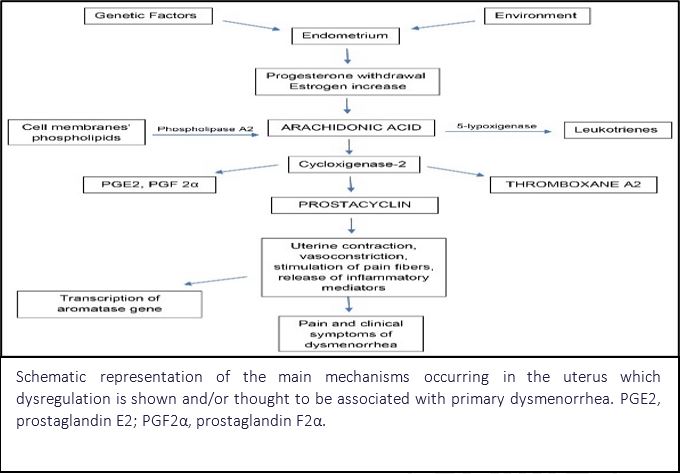
Background: Dysmenorrhea is a common gynecological condition affecting women of reproductive age, characterized by painful menstrual cramps that can significantly impact daily activities and quality of life. Managing dysmenorrhea requires a personalized approach that often combines lifestyle changes, pain management strategies, and pharmaceutical treatments. While NSAIDs and hormonal therapies remain standard treatments, alternative options such as herbal formulations are being explored. M2-Tone Tablet, a polyherbal product by Charak Pharma Pvt. Ltd., was evaluated for efficacy and safety in a Phase 3, open-label, multi-centric clinical trial involving 300 women (aged 18–45) diagnosed with dysmenorrhea.
Materials & Method: This phase 3, prospective, open-label, multi-centric clinical trial aimed to evaluate the clinical efficacy and safety of M2-Tone Tablet, a polyherbal formulation, in managing pelvic pain and dysmenorrhea in 300 women aged 18–45 years diagnosed with dysmenorrhea.
Observation: This clinical trial evaluated the efficacy of M2-Tone Tablet in managing dysmenorrhea over a 90-day period using a verbal multi-dimensional scoring system. A total of 300 participants were assessed for working ability, systemic symptoms, and analgesic use. At baseline, Grade 3 severity was reported by 48% (working ability), 42% (systemic symptoms), and 68% (analgesic use). Post-treatment, these rates declined significantly by 75%, 78.6%, and 73.5%, respectively (p < 0.001). Haematological parameters showed improvement, with haemoglobin increasing from 10.58 ± 2.07 g/dL to 11.47 ± 2.17 g/dL. Menstrual indicators such as clot passage, bleeding duration, and pad usage also reduced. Pain severity, measured on the Visual Analog Scale, improved by 31.2%, with notable relief in menstrual cramps (50.8%), headache (55%), and gastrointestinal symptoms (≥60%).
Result: Overall, M2-Tone tablets demonstrated significant efficacy in managing dysmenorrhea, enhancing functional capacity and reducing analgesic dependence. No adverse events were reported, reinforcing its safety profile as a non-hormonal, herbal alternative for symptom relief.
Downloads
References
Ju H, Jones M, Mishra G. The prevalence and risk factors of dysmenorrhea. Epidemiol Rev. 2014;36(1):104–13.
Itani R, Soubra L, Karout S, Rahme D, Karout L, Khojah HMJ. Primary dysmenorrhea: pathophysiology, diagnosis, and treatment updates. Korean J Fam Med. 2022;43(2):101–8.
Ju H, Jones M, Mishra G. The prevalence and risk factors of dysmenorrhea. Epidemiol Rev. 2014;36(1):104–13.
Francavilla R, Petraroli M, Messina G, Stanyevic B, Bellani AM, Esposito SMR, Street ME. Dysmenorrhea: epidemiology, causes and current state of the art for treatment. Clin Exp Obstet Gynecol. 2023;50(12):274.
Francavilla R, Petraroli M, Messina G, Stanyevic B, Bellani AM, Esposito SMR, Street ME. Dysmenorrhea: epidemiology, causes and current state of the art for treatment. Clin Exp Obstet Gynecol. 2023;50(12):274.
Andersch B, Milsom I. An epidemiologic study of young women with dysmenorrhea. Am J Obstet Gynecol. 1982;144(6):655–60.
Nyeem M. Ashoka (Saraca indica) as women friendly plant: a review. Int J Adv Educ Res. 2017;3:3–7.
Johnson T, Krishnakumar K, Dineshkumar B. Phyto-pharmacological review of Symplocos racemosa bark. J Bio Innov. 2018;7(4):611–7.
Singh J. Shivlingi Beej (Seeds) Benefits, Uses, Dosage & Side Effects [Internet]. Ayur Times; 2017 [cited 2025 Jul 7]. Available from: https://www.ayurtimes.com/shivlingi-beej-seeds/
Thakur S, Kaurav H, Chaudhary G. Shatavari (Asparagus racemosus) – the best female reproductive tonic. Int J Res Rev. 2021;8:73–84.