A Prospective, Open-Label Non-Randomized Clinical Trial to Evaluate the Safety and Efficacy of Sumenta Tablets in Treatment of Anxiety
DOI:
https://doi.org/10.21760/jaims.10.7.8Keywords:
Sumenta Tablet, Anxiety, HAM-A scale, mental health conditions, Generalized anxiety disorder (GAD)Abstract
Background: Anxiety disorders, including GAD, panic disorder, phobias, OCD, PTSD, and social anxiety, significantly impact mood, cognition, and daily functioning. With a global prevalence of 3,895 cases per 100,000 in 2019, these disorders are more common in women and highly prevalent in regions like Latin America and North America. Conventional treatments primarily include SSRIs, SNRIs, and cognitive-behavioural therapy (CBT), though limitations like side effects and treatment resistance necessitate alternative options.
Materials and Methods: This phase 3, prospective, open-label, multi-centric clinical trial assessed the efficacy and safety of Sumenta Tablets in managing anxiety. Conducted per Good Clinical Practice (GCP) guidelines, the study included 150 participants diagnosed with primary anxiety disorder.
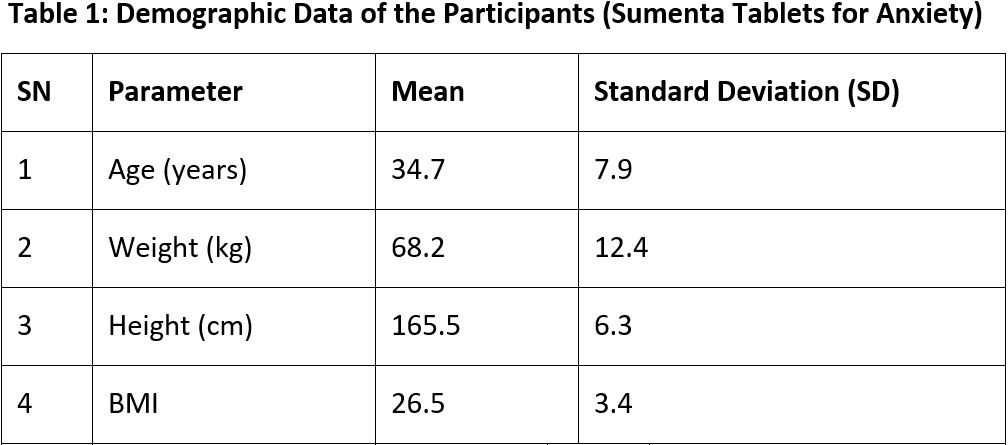
Study Design: A non-randomized, prospective, open-label, multi-centric, trial evaluated the safety and efficacy of Sumenta Tablets in anxiety disorder patients. The study enrolled 150 participants who provided informed consent. Baseline assessments included medical history, psychiatric evaluations (HAM-A scale), and laboratory tests for liver and renal function. Efficacy was measured through anxiety symptom reduction using HAM-A and secondary outcomes such as sleep disturbances, irritability, and quality of life.
Results: Sumenta Tablets significantly reduced anxiety levels, as evidenced by a substantial decrease in the HAM-A score (23.5 ± 5.8 to 14.2 ± 4.1, p < 0.001), and also improved depression scores (BDI: 16.8 ± 6.3 to 10.4 ± 4.5, p < 0.01). Sleep quality improved (Pittsburgh Sleep Scale: 8.5 ± 3.2 to 5.0 ± 2.1, p < 0.001), and cognitive function increased (MoCA: 25.4 ± 3.5 to 27.1 ± 2.9, p = 0.02). Physical activity levels rose (120 ± 55.4 to 180 ± 70.2 minutes/week, p < 0.05), and fatigue and muscle tension decreased significantly. Patient-reported quality of life increased (EQ-5D: 53.2 ± 8.4 to 72.3 ± 9.5, p < 0.001). Biologically, serum cortisol (18.4 ± 5.2 to 12.1 ± 4.3 μg/dL, p = 0.03), vitamin D (14.6 ± 5.7 to 21.8 ± 6.3 mg/dL, p = 0.02), and inflammatory markers (CRP: 6.8 ± 2.3 to 4.0 ± 1.7 mg/L, p = 0.01) all improved. Patient satisfaction increased significantly (VAS: 4.2 ± 1.5 to 8.5 ± 1.2, p < 0.001), indicating high treatment acceptability and effectiveness.
Downloads
References
Adwas A, Jbireal J, Azab A. Anxiety: insights into signs, symptoms, etiology, pathophysiology, and treatment. S Afr J Med Sci. 2019;2:80–91.
Javaid S, Hashim I, Hashim M, Samad M, Ahbabi A. Epidemiology of anxiety disorders: global burden and sociodemographic associations. Middle East Curr Psychiatry. 2023;30. doi:10.1186/s43045-023-00315-3
Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 2015 Sep;17(3):327–35. doi:10.31887/DCNS.2015.17.3/bbandelow. PMID: 26487813; PMCID: PMC4610617
Adwas A, Jbireal J, Azab A. Anxiety: insights into signs, symptoms, etiology, pathophysiology, and treatment. S Afr J Med Sci. 2019;2:80–91.
Penninx BW, Pine DS, Holmes EA, Reif A. Anxiety disorders. Lancet. 2021 Mar 6;397(10277):914–27. doi:10.1016/S0140-6736(21)00359-7. PMID: 33581801; PMCID: PMC9248771
Curtiss JE, Levine DS, Ander I, Baker AW. Cognitive-behavioral treatments for anxiety and stress-related disorders. Focus (Am Psychiatr Publ). 2021 Jun;19(2):184–9. doi:10.1176/appi.focus.20200045. PMID: 34690581; PMCID: PMC8475916
Otte C. Cognitive behavioral therapy in anxiety disorders: current state of the evidence. Dialogues Clin Neurosci. 2011;13(4):413–21. doi:10.31887/DCNS.2011.13.4/cotte. PMID: 22275847; PMCID: PMC3263389
Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci. 2017 Jun;19(2):93–107. doi:10.31887/DCNS.2017.19.2/bbandelow. PMID: 28867934; PMCID: PMC5573566
Lopresti AL, Smith SJ, Ali S, Metse AP, Kalns J, Drummond PD. Effects of a Bacopa monnieri extract (Bacognize®) on stress, fatigue, quality of life and sleep in adults with self-reported poor sleep: a randomised, double-blind, placebo-controlled study. J Funct Foods. 2021;85:104671. doi:10.1016/j.jff.2021.104671
Eraiah MM, Shekhar HC, Joshua L, Thomas JV. Effect of Bacopa monnieri extract on memory and cognitive skills in adult humans: a randomized, double-blind, placebo-controlled study. J Psychiatry Cogn Behav. 2024;8:168. doi:10.29011/2574-7762.000068
Esfandiari E, Ghanadian M, Rashidi B, Mokhtarian A, Vatankhah AM. The effects of Acorus calamus L. in preventing memory loss, anxiety, and oxidative stress on lipopolysaccharide-induced neuroinflammation rat models. Int J Prev Med. 2018;9(1):85. doi:10.4103/ijpvm.IJPVM_75_18
Rai AR, Joy T, Poojari M, Pai MM, Massand A, Murlimanju BV. Role of Acorus calamus in preventing depression, anxiety, and oxidative stress in long-term socially isolated rats. Vet World. 2023 Aug;16(8):1755–64. doi:10.14202/vetworld.2023.1755-1764. PMID: 37766700; PMCID: PMC10521175
Gamne R, Misar S, Rai M. Evaluation of comparative efficacy of Celastrus paniculatus (Jyotishmati) capsule versus sertraline capsule in the management of Chittodvega (generalized anxiety disorder): protocol for a randomized controlled trial. F1000Res. 2024 Oct 28;12:1577. doi:10.12688/f1000research.139473.2. PMID: 39114320; PMCID: PMC11305454
Tyagi T, Sharma S, Sharma R. Pharmacological actions of Valeriana wallichii (Tagara): a fundamental analysis supporting traditional benefits. Int J Ayurveda Pharma Res. 2022;10(Suppl 1):1–7. doi:10.47070/ijapr.v10iSuppl1.2468